Jun 24, 2023To calculate the value of k at 298 K for the given reaction, we need to use the equation for the equilibrium constant, which is expressed as the ratio of the concentrations of the products to the concentrations of the reactants, each raised to their respective stoichiometric coefficients.
10.+15.2+Spontaneity+and+Gibbs+free+energy | PDF | Gibbs Free Energy | Chemical Reactions
Enter your answers in scientifie notation Part 1: (a) Calculate K at 298 K for the following reaction: 2C (graphite) + O2 (g) 2CO (g) Ao) C (graphite) 0 COg)137.2 02 (g) K=1.25 ×1048 Part 2 out of 2 0 (b) Use the equilibrium constant to calculate AG (in kJ) at 298 K for the following reaction: 2HCIg)Br20) 2HBr (g)+C2g) K-2.22x 1015 at

Source Image: m.youtube.com
Download Image
Nov 24, 2022This page titled 4.4: Calculations of Enthalpies of Reaction at T ≠ 298 K is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Roberto Peverati via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request.

Source Image: youtube.com
Download Image
Calculate the equilibrium constant for the following reaction at `298K` and `1` atmospheric pres… – YouTube Sep 2, 2023Expert-Verified Answer question No one rated this answer yet — why not be the first? 😎 mathewdse34 Final answer: The equilibrium constant for the given reaction at 298K can be calculated using the formula k = e^ (-ΔG/RT). Taking into account the data provided in the question, the ΔG value calculates to -34600 Joules.

Source Image: m.youtube.com
Download Image
Calculate K At 298 K For The Following Reaction
Sep 2, 2023Expert-Verified Answer question No one rated this answer yet — why not be the first? 😎 mathewdse34 Final answer: The equilibrium constant for the given reaction at 298K can be calculated using the formula k = e^ (-ΔG/RT). Taking into account the data provided in the question, the ΔG value calculates to -34600 Joules. Jun 19, 2023To calculate the equilibrium constant (K) at 298 K for the reaction involving strontium sulfate (SrSO4), we need the balanced chemical equation. SrSO4(s) ⇌ SrO(s) + SO2(g) In this reaction, strontium sulfate decomposes into strontium oxide and sulfur dioxide. Now, let’s proceed with the calculation of K at 298 K.
Find the equilibrium constant at `298K` for the reaction, `Cu^(2+)(aq) +In^(2+)(aq)hArr Cu^ – YouTube
The relationship shown in Equation 15.2.5 is true for any pair of opposing reactions regardless of the mechanism of the reaction or the number of steps in the mechanism. The equilibrium constant can vary over a wide range of values. The values of K shown in Table 15.2.2, for example, vary by 60 orders of magnitude. Calculate ΔG° at 298 K for these reactions and predict the effect… | Channels for Pearson+

Source Image: pearson.com
Download Image
Solved Calculate K at 298 K for the following reaction: 2 | Chegg.com The relationship shown in Equation 15.2.5 is true for any pair of opposing reactions regardless of the mechanism of the reaction or the number of steps in the mechanism. The equilibrium constant can vary over a wide range of values. The values of K shown in Table 15.2.2, for example, vary by 60 orders of magnitude.
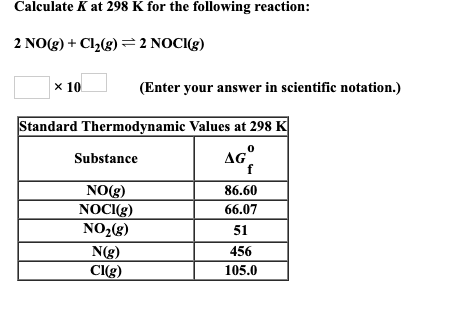
Source Image: chegg.com
Download Image
10.+15.2+Spontaneity+and+Gibbs+free+energy | PDF | Gibbs Free Energy | Chemical Reactions Jun 24, 2023To calculate the value of k at 298 K for the given reaction, we need to use the equation for the equilibrium constant, which is expressed as the ratio of the concentrations of the products to the concentrations of the reactants, each raised to their respective stoichiometric coefficients.

Source Image: scribd.com
Download Image
Calculate the equilibrium constant for the following reaction at `298K` and `1` atmospheric pres… – YouTube Nov 24, 2022This page titled 4.4: Calculations of Enthalpies of Reaction at T ≠ 298 K is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Roberto Peverati via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request.

Source Image: youtube.com
Download Image
Solved Part A Calculate the equilibrium constant K for the | Chegg.com Calculating K For Overall Reaction 15m. Le Chatelier’s Principle 20m. ICE Charts 30m. Reaction Quotient 15m. 17. Acid and Base Equilibrium 5h 4m. Worksheet. Acids Introduction 9m. … Calculate ΔG° at 298 K for these reactions and predict the effect on ΔG° of lowering the temperature. b. CaCO3(s) ¡ CaO(s) + CO2( g)
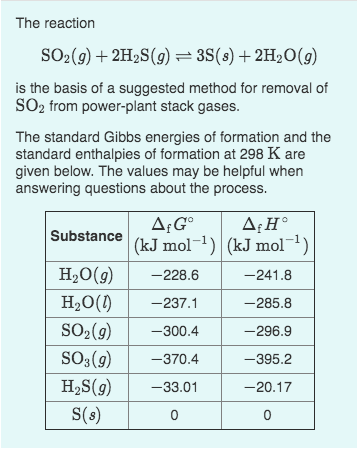
Source Image: chegg.com
Download Image
Calculate the equilibrium constant for the following reaction at 298 K and 1 atm pressure : … – YouTube Sep 2, 2023Expert-Verified Answer question No one rated this answer yet — why not be the first? 😎 mathewdse34 Final answer: The equilibrium constant for the given reaction at 298K can be calculated using the formula k = e^ (-ΔG/RT). Taking into account the data provided in the question, the ΔG value calculates to -34600 Joules.

Source Image: m.youtube.com
Download Image
Find out the value of equilibrium constant for the following reaction at 298 K…… – YouTube Jun 19, 2023To calculate the equilibrium constant (K) at 298 K for the reaction involving strontium sulfate (SrSO4), we need the balanced chemical equation. SrSO4(s) ⇌ SrO(s) + SO2(g) In this reaction, strontium sulfate decomposes into strontium oxide and sulfur dioxide. Now, let’s proceed with the calculation of K at 298 K.

Source Image: m.youtube.com
Download Image
Solved Calculate K at 298 K for the following reaction: 2 | Chegg.com
Find out the value of equilibrium constant for the following reaction at 298 K…… – YouTube Enter your answers in scientifie notation Part 1: (a) Calculate K at 298 K for the following reaction: 2C (graphite) + O2 (g) 2CO (g) Ao) C (graphite) 0 COg)137.2 02 (g) K=1.25 ×1048 Part 2 out of 2 0 (b) Use the equilibrium constant to calculate AG (in kJ) at 298 K for the following reaction: 2HCIg)Br20) 2HBr (g)+C2g) K-2.22x 1015 at
Calculate the equilibrium constant for the following reaction at `298K` and `1` atmospheric pres… – YouTube Calculate the equilibrium constant for the following reaction at 298 K and 1 atm pressure : … – YouTube Calculating K For Overall Reaction 15m. Le Chatelier’s Principle 20m. ICE Charts 30m. Reaction Quotient 15m. 17. Acid and Base Equilibrium 5h 4m. Worksheet. Acids Introduction 9m. … Calculate ΔG° at 298 K for these reactions and predict the effect on ΔG° of lowering the temperature. b. CaCO3(s) ¡ CaO(s) + CO2( g)