Feb 8, 2023A flask contains equal masses of F2 and Cl2 with a total pressure of 1.94 atm at 298K. What is the partial pressure (in atm) of Cl2 in the flask? Round your answer up to 2 decimal places. Video Answer. Solved by verified expert Created on Feb. 8, 2023, 10:57 p.m. Video Answers to Similar Questions
SOLVED: A flask contains equal masses of F2 and Cl2 with a total pressure of 1.94 atm at 298K. What is the partial pressure (in atm) of Cl2 in the flask? Round
Mar 6, 2023Faq Physics A flask contains equal masses of f2 and cl2 with a total pressure of 2.00 atm at 298k. What is the partial pressure of cl2 in the flask? Step-by-step answer P Answered by Master Partial Pressure of F₂ = 1.30 atm Partial pressure of Cl₂ = 0.70 atm Explanation: Partial pressure for gases are given by Daltons law.
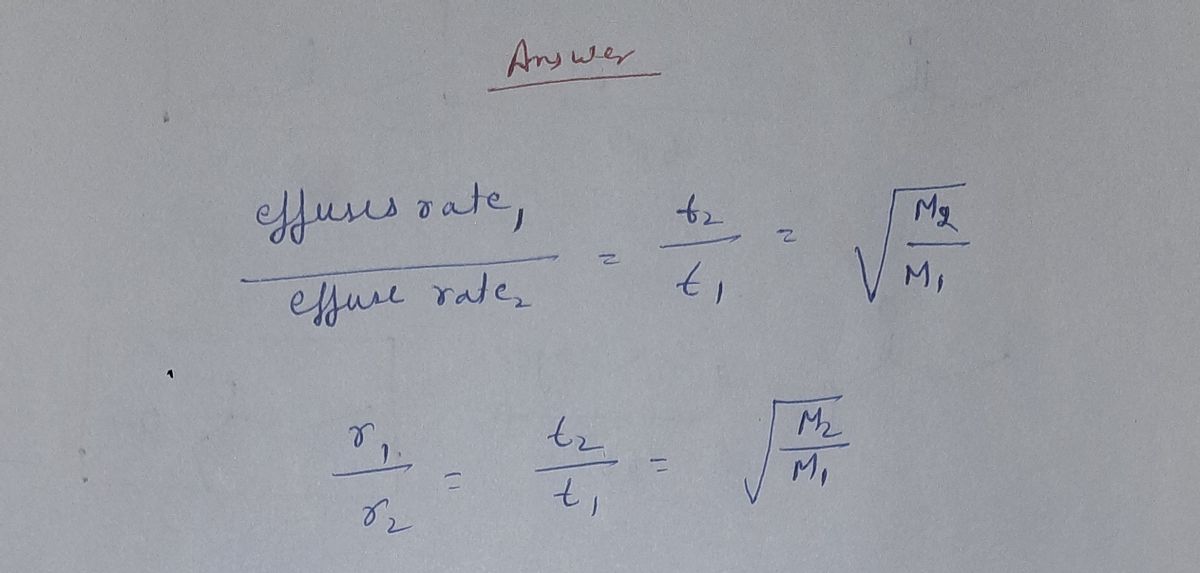
Source Image: bartleby.com
Download Image
If the flask is then cooled to room temperature, the gas condenses and the mass of the gas that filled the flask, and is now liquid, can be measured. (credit: modification of work by Mark Ott) Using this procedure, a sample of chloroform gas weighing 0.494 g is collected in a flask with a volume of 129 cm 3 at 99.6 °C when the atmospheric

Source Image: scribd.com
Download Image
Part – I: Subjective Questions: Mole Concept | PDF | Mass Concentration (Chemistry) | Mass Fraction (Chemistry) Since there is an equal number of each element in the reactants and products of F2 + 2KCl = 2KF + Cl2, the equation is balanced. Balance F2 + KCl = KF + Cl2 Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction as at

Source Image: m.youtube.com
Download Image
A Flask Contains Equal Masses Of F2 And Cl2
Since there is an equal number of each element in the reactants and products of F2 + 2KCl = 2KF + Cl2, the equation is balanced. Balance F2 + KCl = KF + Cl2 Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction as at Science Chemistry Quiz 5 Chemistry Gases 5.0 (1 review) Get a hint Four identical 1.0-L flasks contain the gases He, Cl2, CH4, and NH3, each at 0°C and 1 atm pressure. Which gas sample has the greatest number of molecules? Cl2 NH3 He All the gases have the same number of molecules. CH4 Click the card to flip 👆
A flask contains argon and chlorine in the ratio 2:1 by mass. The temperature of the mixture is … – YouTube
Answer 1 day ago Answer To solve this problem, we need to use the concept of partial pressures and the ideal gas law. The ideal gas law states that the pressure of a gas is directly proportional to its number of moles. Ideal Gas | PDF

Source Image: scribd.com
Download Image
1-ChapteR-Short-Question-With-Answer (20 Files Merged) PDF | PDF Answer 1 day ago Answer To solve this problem, we need to use the concept of partial pressures and the ideal gas law. The ideal gas law states that the pressure of a gas is directly proportional to its number of moles.

Source Image: scribd.com
Download Image
SOLVED: A flask contains equal masses of F2 and Cl2 with a total pressure of 1.94 atm at 298K. What is the partial pressure (in atm) of Cl2 in the flask? Round Feb 8, 2023A flask contains equal masses of F2 and Cl2 with a total pressure of 1.94 atm at 298K. What is the partial pressure (in atm) of Cl2 in the flask? Round your answer up to 2 decimal places. Video Answer. Solved by verified expert Created on Feb. 8, 2023, 10:57 p.m. Video Answers to Similar Questions
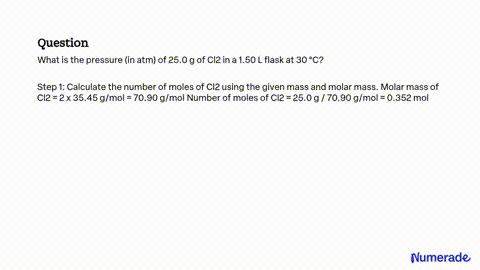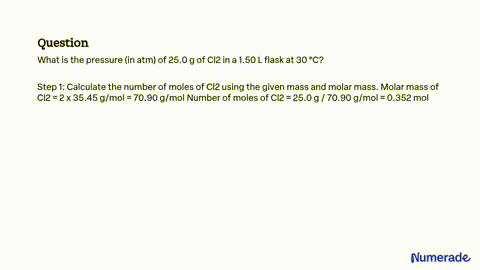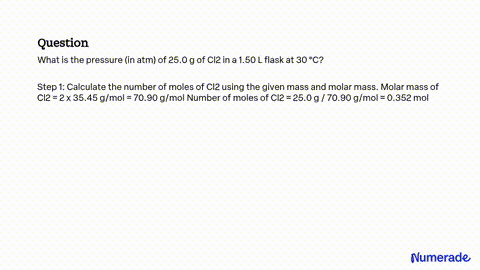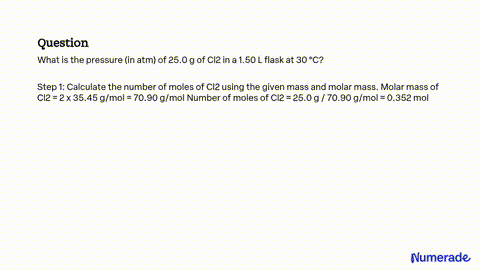
Source Image: numerade.com
Download Image
Part – I: Subjective Questions: Mole Concept | PDF | Mass Concentration (Chemistry) | Mass Fraction (Chemistry) If the flask is then cooled to room temperature, the gas condenses and the mass of the gas that filled the flask, and is now liquid, can be measured. (credit: modification of work by Mark Ott) Using this procedure, a sample of chloroform gas weighing 0.494 g is collected in a flask with a volume of 129 cm 3 at 99.6 °C when the atmospheric

Source Image: scribd.com
Download Image
Solved Data and Observations Mass of foil and flask filled | Chegg.com Question A flask contains equal masses of F 2 and Cl 2 with a total pressure of 1.94 atm at 298K. What is the partial pressure (in atm) of Cl 2 in the flask? Round your answer up to 2 decimal places Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps See solution Check out a sample Q&A here Knowledge Booster

Source Image: chegg.com
Download Image
Answered: There is 12.0 g of carbon dioxide gas… | bartleby Since there is an equal number of each element in the reactants and products of F2 + 2KCl = 2KF + Cl2, the equation is balanced. Balance F2 + KCl = KF + Cl2 Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction as at

Source Image: bartleby.com
Download Image
Part – I: Subjective Questions: Mole Concept | PDF | Mass Concentration (Chemistry) | Mass Fraction (Chemistry) Science Chemistry Quiz 5 Chemistry Gases 5.0 (1 review) Get a hint Four identical 1.0-L flasks contain the gases He, Cl2, CH4, and NH3, each at 0°C and 1 atm pressure. Which gas sample has the greatest number of molecules? Cl2 NH3 He All the gases have the same number of molecules. CH4 Click the card to flip 👆

Source Image: scribd.com
Download Image
1-ChapteR-Short-Question-With-Answer (20 Files Merged) PDF | PDF
Part – I: Subjective Questions: Mole Concept | PDF | Mass Concentration (Chemistry) | Mass Fraction (Chemistry) Mar 6, 2023Faq Physics A flask contains equal masses of f2 and cl2 with a total pressure of 2.00 atm at 298k. What is the partial pressure of cl2 in the flask? Step-by-step answer P Answered by Master Partial Pressure of F₂ = 1.30 atm Partial pressure of Cl₂ = 0.70 atm Explanation: Partial pressure for gases are given by Daltons law.
Part – I: Subjective Questions: Mole Concept | PDF | Mass Concentration (Chemistry) | Mass Fraction (Chemistry) Answered: There is 12.0 g of carbon dioxide gas… | bartleby Question A flask contains equal masses of F 2 and Cl 2 with a total pressure of 1.94 atm at 298K. What is the partial pressure (in atm) of Cl 2 in the flask? Round your answer up to 2 decimal places Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps See solution Check out a sample Q&A here Knowledge Booster