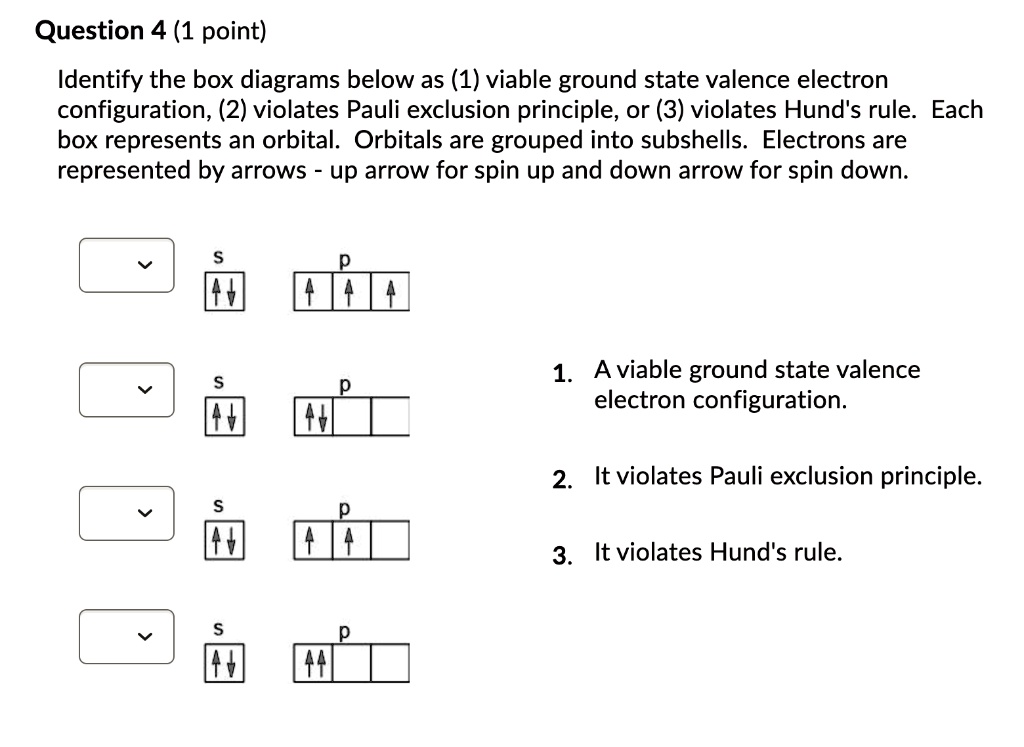
Step 1 The Pauli Exclusion Principle is a fundamental concept in quantum mechanics, named after the Austria… View the full answer Step 2 Final answer Previous question Next question Transcribed image text: Which orbital-filling diagram violates the Pauli exclusion principle?
SOLVED: He filled with electrons; In the images, each orbital is represented by subshells of an atom. The orbital diagram showing or violating the Pauli exclusion principle is represented by half an
Essentially, the implication of Pauli’s exclusion principle is that when two electrons are in the same orbital, they must have opposite spins. In other words, you should put the arrows representing the electrons in opposite directions. For example, the electron configuration of boron is 1 s2 2 s2 2 p1 which can be represented by orbital diagrams:
Source Image: numerade.com
Download Image
Jan 30, 2023The Pauli Exclusion Principle states that, in an atom or molecule, no two electrons can have the same four electronic quantum numbers. As an orbital can contain a maximum of only two electrons, the two electrons must have opposing spins.
Source Image: chegg.com
Download Image
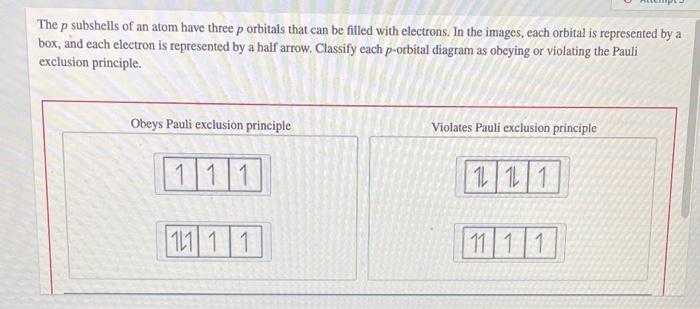
Solved The p subshells of an atom have three p orbitaIs that | Chegg.com Figure 3.8.3 3.8. 3: Orbital filling diagram for carbon. Oxygen has four 2p 2 p electrons. After each 2p 2 p orbital has one electron in it, the fourth electron can be placed in the first 2p 2 p orbital with a spin opposite that of the other electron in that orbital. Figure 3.8.4 3.8. 4: Orbital filling diagram for oxygen.

Source Image: brainly.com
Download Image
Which Orbital-Filling Diagram Violates The Pauli Exclusion Principle
Figure 3.8.3 3.8. 3: Orbital filling diagram for carbon. Oxygen has four 2p 2 p electrons. After each 2p 2 p orbital has one electron in it, the fourth electron can be placed in the first 2p 2 p orbital with a spin opposite that of the other electron in that orbital. Figure 3.8.4 3.8. 4: Orbital filling diagram for oxygen. Expert Answer 1. Option B is the correct answer. Because firstly each degenerate orbital … View the full answer Transcribed image text: Which orbital-filling diagram violates the Pauli exclusion principle?
HELP PLSSS Consider the orbital diagram shown. Which electron rule is broken in the diagram? A. Aufbau – brainly.com
Science Chemistry Chemistry questions and answers Which orbital-filling diagram violates the Pauli exclusion principle? This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Which orbital-filling diagram violates the Pauli exclusion principle? 16 Pauli Exclusion Principle Images, Stock Photos, 3D objects, & Vectors | Shutterstock

Source Image: shutterstock.com
Download Image
Solved please answer asap I just have 20 minutes to submit | Chegg.com Science Chemistry Chemistry questions and answers Which orbital-filling diagram violates the Pauli exclusion principle? This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Which orbital-filling diagram violates the Pauli exclusion principle?

Source Image: chegg.com
Download Image
SOLVED: He filled with electrons; In the images, each orbital is represented by subshells of an atom. The orbital diagram showing or violating the Pauli exclusion principle is represented by half an Step 1 The Pauli Exclusion Principle is a fundamental concept in quantum mechanics, named after the Austria… View the full answer Step 2 Final answer Previous question Next question Transcribed image text: Which orbital-filling diagram violates the Pauli exclusion principle?
Source Image: numerade.com
Download Image
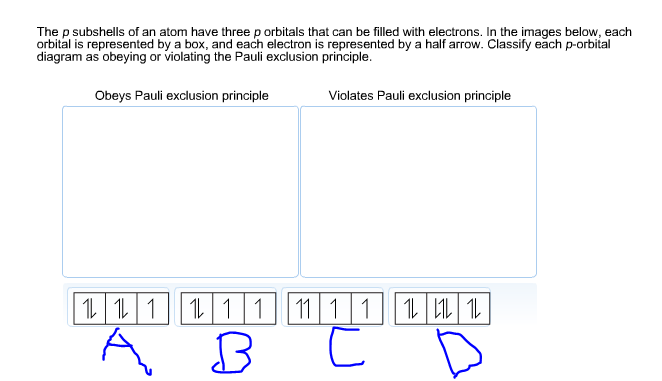
Solved The p subshells of an atom have three p orbitaIs that | Chegg.com Jan 30, 2023The Pauli Exclusion Principle states that, in an atom or molecule, no two electrons can have the same four electronic quantum numbers. As an orbital can contain a maximum of only two electrons, the two electrons must have opposing spins.
Source Image: chegg.com
Download Image
Pauli’s Exclusion Principle | Pauli exclusion principle, Chemistry lessons, Aufbau principle The Pauli exclusion principle, which states that no two electrons in an atom can have the same set of four quantum numbers. … Figure \(\PageIndex2\): In an orbital filling diagram, a square represents an orbital, while arrows represent electrons. An arrow pointing upward represents one spin direction, while an arrow pointing downward

Source Image: pinterest.com
Download Image
SOLVED: Which orbital-filling diagram violates the Pauli exclusion principle? A [Ar] 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 B [Ar] 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^1 C [Ar] 1s^2 2s^2 2p^6 Figure 3.8.3 3.8. 3: Orbital filling diagram for carbon. Oxygen has four 2p 2 p electrons. After each 2p 2 p orbital has one electron in it, the fourth electron can be placed in the first 2p 2 p orbital with a spin opposite that of the other electron in that orbital. Figure 3.8.4 3.8. 4: Orbital filling diagram for oxygen.
![SOLVED: Which orbital-filling diagram violates the Pauli exclusion principle? A [Ar] 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 B [Ar] 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^1 C [Ar] 1s^2 2s^2 2p^6](https://cdn.numerade.com/project-universal/previews/bc169bd3-8788-47af-9a27-fadda8c520f1.gif)
Source Image: numerade.com
Download Image
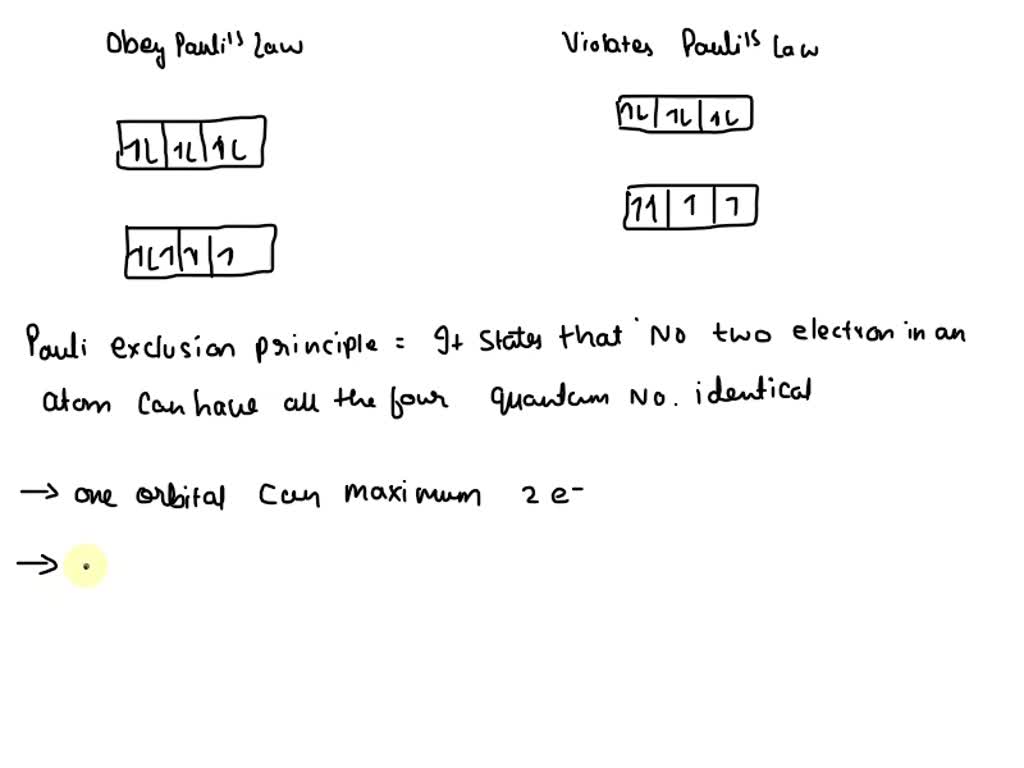
SOLVED: VIII Shown below are portions of orbital diagrams representing the ground-state electron configuration certain elements. Which of them violate the Pauli exclusion principle? Hund s rule? Explain. ( 12 pts) Expert Answer 1. Option B is the correct answer. Because firstly each degenerate orbital … View the full answer Transcribed image text: Which orbital-filling diagram violates the Pauli exclusion principle?
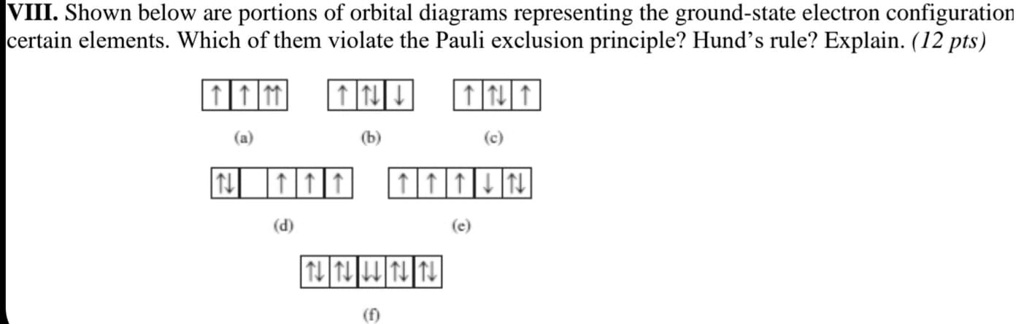
Source Image: numerade.com
Download Image
Solved please answer asap I just have 20 minutes to submit | Chegg.com
SOLVED: VIII Shown below are portions of orbital diagrams representing the ground-state electron configuration certain elements. Which of them violate the Pauli exclusion principle? Hund s rule? Explain. ( 12 pts) Essentially, the implication of Pauli’s exclusion principle is that when two electrons are in the same orbital, they must have opposite spins. In other words, you should put the arrows representing the electrons in opposite directions. For example, the electron configuration of boron is 1 s2 2 s2 2 p1 which can be represented by orbital diagrams:
Solved The p subshells of an atom have three p orbitaIs that | Chegg.com SOLVED: Which orbital-filling diagram violates the Pauli exclusion principle? A [Ar] 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 B [Ar] 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^1 C [Ar] 1s^2 2s^2 2p^6 The Pauli exclusion principle, which states that no two electrons in an atom can have the same set of four quantum numbers. … Figure \(\PageIndex2\): In an orbital filling diagram, a square represents an orbital, while arrows represent electrons. An arrow pointing upward represents one spin direction, while an arrow pointing downward